Half-life of GATTEX in patients with SBS1,2
~1.3 HOURS
(AGE: >17 YEARS)
The information in the site you are about to see is intended for US healthcare professionals only. Visit Patient Site.
LYNDA,
GATTEX PATIENT
PEGGY,
GATTEX PATIENT

The ability of GATTEX (teduglutide) to improve intestinal absorption was studied in 17 adult subjects with SBS (n=2-3 per dose group) using daily doses of 0.03, 0.1, or 0.15 mg/kg (doses ranging from 0.6 to 3 times the recommended dose) in a 21-day, open-label, multicenter, dose-ranging study. Fourteen of 17 patients were dependent on parenteral support (PS). All subcutaneous (abdomen) doses studied, except 0.03 mg/kg once daily, resulted in enhanced gastrointestinal fluid (wet weight) absorption of approximately 750 to 1000 mL/day, and increased villus height and crypt depth of the intestinal mucosa.1
The recommended dosage of GATTEX for both adult and pediatric patients is 0.05 mg/kg once daily by subcutaneous injection. The recommended dosage in adult and pediatric patients with moderate and severe renal impairment and end-stage renal disease (estimated glomerular filtration rate [eGFR] <60 mL /min/1.73 m2) is 0.025 mg /kg once daily.1
Share the video below with your patients to see how GATTEX works.

“My surgeon had heard about GATTEX, and he explained that this drug might help me reduce the amount of volume and days on total parenteral nutrition (TPN).”
| Average | Range | |
|---|---|---|
| Age | 50 years | 18-82 years |
| Estimated small bowel length |
77.3 cm | 5-343 cm |
| Length of time on PS | 6 years | 1-26 years |
| Prescribed days per week on PS |
5.73 days | 3-7 days |
| Infusion volume | 13 L/week | 0.9-35 L/week |
Patients had varying types of intestinal resection1,7
The most common reasons for intestinal resection included1:
See how to get your appropriate patients started, or keep reading to explore results with GATTEX, safety profile, dosing, time to response, and weaning PS.
Adult patients achieved significant reductions in parenteral support (PS) volume with GATTEX1*
*The primary endpoint in STEPS was defined as a patient achieving a ≥20% reduction in weekly PS volume from baseline (immediately before randomization) to Weeks 20 and 24. Clinical response in STEPS-2 was defined as a ≥20% reduction in weekly PS volume from baseline (start of GATTEX treatment) to the last visit.1,6
Patients who received GATTEX achieved an average reduction of 4.4 L/week.¶
Patients who received placebo achieved an average reduction of 2.3 L/week.‖
After 30 months, patients treated with GATTEX (n=30) achieved a mean reduction in PS volume of 7.55 L/week, which represented a 66% reduction from baseline.
-66%
§In STEPS, mean reduction in PS volume was a selected secondary endpoint.
¶From a pretreatment baseline of 12.9 L/week (n=43).
‖From a pretreatment baseline of 13.2 L/week (n=43).

“With the help of GATTEX, my doctor was able to cut my weekly PS. I'm now infusing fewer liters of PS a week.”
1 out of 3 adult patients achieved complete freedom from parenteral support (PS)1
††During the study, patients were maintained on GATTEX after weaning off of PS. Prior to GATTEX, these 10 patients required 3.5-13.4 L/week of PS for 1.2-15.5 years.

“Now, I spend my days off PS engaged in pastimes like being with friends, traveling, and engaging in volunteer activities.”
Bowel anatomy may impact time to response
Post hoc analysis8
A Takeda-sponsored post hoc analysis of STEPS and STEPS-2 identified factors that may be associated with mean time to sustain parenteral support (PS) reduction.
To evaluate factors associated with sustained PS volume reduction and early vs late response, a Takeda-sponsored post hoc analysis was conducted of patients with SBS who were treated with GATTEX in the STEPS study (n=43) and who then continued treatment with GATTEX for up to 24 months in the extension study, STEPS-2.¶¶¶
Early responders (n=27)
3.6
MonthsLate responders (n=7)
10.0
MonthsEarly responders
Late responders
Study limitations: This study was limited by the relatively small sample size, which may not have sufficient statistical power to identify factors associated with response. This study was also limited by the heterogeneity within the group of patients who had SBS with intestinal failure. The population consisted of patients who were subject to specific inclusion and exclusion criteria. Thus, the results may have limited generalizability.
| CHARACTERISTICS | EARLY RESPONDERS (n-27) |
LATE RESPONDERS (n=7) |
P VALUE |
|---|---|---|---|
| Baseline demographics | |||
| Age (years), mean (SD) | 51.6 (13.3) | 52.4 (10.2) | 0.966 |
| Male, n (%) | 14 (51.9) | 3 (42.9) | 0.672 |
| White, n (%) | 26 (96.3) | 7 (100.0) | 0.605 |
| BMI (kg/m2), mean (SD) | 22.3 (3.3) | 22.3 (3.9) | 0.881 |
| Baseline PS characteristics, mean (SD) | |||
| Composite fluid balance (L/week) | 17.3 (11.7) | 18.3 (15.0) | 0.966 |
| Actual baseline PS volume (L/week) | 13.5 (7.7) | 12.7 (9.8) | 0.639 |
| Time since start on PS dependence (years) | 7.4 (6.4) | 3.5 (3.0) | 0.287 |
| Actual number of days of PS per week | 5.8 (1.5) | 5.4 (1.8) | 0.537 |
| Concomitant medications | |||
| Narcotics, n (%) | 10 (37.0) | 3 (42.9) | 1.000 |
| Baseline SBS characteristics | |||
| Cause of major intestinal resection, n (%) | |||
| Crohn's disease | 7 (25.9) | 1 (14.3) | 1.000 |
| Vascular disease | 8 (29.6) | 4 (57.1) | 0.211 |
| Injury | 2 (7.4) | 1 (14.3) | 0.511 |
| Volvulus | 3 (11.1) | - | 1.000 |
| Cancer | 1 (3.7) | - | 1.000 |
| Other | 6 (22.2) | 1 (14.3) | 1.000 |
| Presence of stoma, n (%) | 15 (55.6) | 2 (28.6) | 0.203 |
| Type of stoma, n (%) | |||
| Jejunostomy | 10 (37.0) | - | 0.078 |
| Ileostomy | 3 (11.1) | - | 1.000 |
| Colostomy | 2 (7.4) | 2 (28.6) | 0.180 |
| Missing or not available | 12 (44.4) | 5 (71.4) | 0.398 |
| Colon-in-continuity, n (%) | 14 (51.9) | 7 (100.0) | 0.020‖‖‖ |
| Percentage of colon remaining, mean (SD) | 24.6 (26.1) | 57.1 (19.8) | 0.016‖‖‖ |
| Estimated remaining small intestine length (cm), n (%) | |||
| <60 cm | 11 (40.7) | 3 (42.9) | 1.000 |
| ≥60 cm | 14 (51.9) | 3 (42.9) | 1.000 |
| Missing | 2 (7.4) | 1 (14.3) | 0.511 |
| Presence of distal/terminal ileum, n (%) | 5 (18.5) | 3 (42.9) | 0.176 |
| Presence of ileocecal valve, n (%) | 0 (0.0) | 2 (28.6) | 0.004‖‖‖ |
| Time since last small bowel resection (years), mean (SD) | 5.8 (5.1) | 3.9 (2.7) | 0.639 |
Adapted from Joly et al, 2016, United European Gastroenterology Week.
¶¶¶This is a post hoc analysis of patients who completed STEPS and STEPS-2. Please note that efficacy in STEPS was based on the intent-to-treat population, while efficacy in STEPS-2 was based on study completers. Further randomized, controlled clinical studies are necessary to corroborate these findings.8-10
‖‖‖P<0.05.
| Ongoing | Every 6 months |
After 1 year of GATTEX* |
At least every 5 years |
||
|---|---|---|---|---|---|
| Colonoscopy1 | Examination of the entire colon with removal of polyps |
|
|
||
|
Laboratory assessments1 |
Bilirubin |
|
|||
| Alkaline phosphatase |
|
||||
| Lipase |
|
||||
| Amylase |
|
||||
| Clinical evaluations1 | Signs and symptoms of intestinal obstruction |
|
|||
| Signs and symptoms of fluid imbalance and fluid overload |
|
||||
| Increased absorption of concomitant oral medication(s) |
|
||||
| Observation of other adverse events |
|
| Colonoscopy after 1 year of GATTEX* and at least every 5 years1 |
|---|
| Examination of the entire colon with removal of polyps |
| Laboratory assessments every 6 months1 |
| Bilirubin |
| Alkaline phosphatase |
| Lipase |
| Amylase |
| Ongoing clinical evaluations1 |
| Signs and symptoms of intestinal obstruction |
| Signs and symptoms of fluid imbalance and fluid overload |
| Increased absorption of concomitant oral medication(s) |
| Observation of other adverse events |
Discontinuation of treatment with GATTEX may result in fluid and electrolyte imbalance. Fluid and electrolyte status should be monitored in patients who discontinue treatment with GATTEX.1
*Follow-up colonoscopy (or alternate imaging) is recommended at the end of 1 year of treatment with GATTEX.1
See how to get your appropriate patients started, or keep reading for information on dosing.

“Over time with GATTEX, my doctors started slowly reducing my PS. A year later, my PS had been stopped altogether.”
Patients with short bowel syndrome (SBS) differ in age and pathophysiology as well as in duration of parenteral support (PS) and nutritional status. These and other factors can impact weaning success.9,11
Multidisciplinary care improves outcomes in weaning parenteral support (PS), facilitating enteral autonomy, and reducing complications. To establish an MDT, the first step is to start with a single dedicated physician who will drive the development of the team. This physician will connect with specialists needed to help support the patient. The size of the team may vary, depending on the circumstances. Start by adding a few specialists at a time who can contribute to the success of the patient’s weaning goals.10,13-15
Assembles team and oversees treatment
strategy
(e.g. gastroenterologist (GI), surgeon,
nephrologist, infectious disease doctor)
Provides infusion support
Collaborates with care team on medications
and PS
Provides PS and GATTEX
Monitors nutrition and hydration
Helps educate patients on PS infusions
and stoma care
Manages overall health and medical plan
Assists patients with challenges and connects
them with the appropriate resources
*While a gastroenterologist is often the leading physician, other physicians could serve in this role as well.
Depending on patient characteristics, eliminating PS requirements may not be possible — but patients may still achieve meaningful reductions in parenteral support (PS)
volume and time, with fewer hours/days on PS9,10,16
Clearly defined care protocols should be established for situations such as dehydration or nutritional instability that may require IV fluids or vitamin/mineral/electrolyte supplements and diet adjustment
Patients should have a good understanding of who is part of their multidisciplinary team, their contact information, and how each of them will help during the weaning process10,13-15
Patients need to remain consistent with all therapies (modified diet, ORS, medications, vitamin/mineral/electrolyte supplements, and use of PS and adherence to PS reductions as directed)
Homemade Oral Rehydration Solution
1 quart of ready to drink Gatorade® G2 Low Calorie*
1/2 teaspoon salt
Directions: Add salt to ready to drink Gatorade G2 and shake well.
Note: Potassium levels in this recipe are well below the recommended amount for an ORS
*Gatorade® is a registered trademark of PepsiCo.
Patients should be nutritionally stable (ie, meet dry weight, steady calorie and protein intake from all sources) and well hydrated before they begin to wean PS. Therefore, it is recommended that steps be taken to optimize diet, oral fluid intake, and antidiarrheal/antisecretory/pain management medications before starting the weaning process to avoid dehydration and malnutrition.
In STEPS and STEPS-2 studies, once a patient was optimized and stable, parenteral support (PS) infusions were slowly reduced over time.5,6 Gradual reductions minimize the risk of dehydration and increase the likelihood of weaning success.10
Nutrition-focused physical assessment
Stool output
Oral intake
Electrolyte and micronutrient levels
Body weight
Monitor electrolytes, trace elements, vitamins and minerals
Antidiarrheal medications should be continued and adjusted based on stool volume (stoma), frequency and consistency
Monitor weight for nutritional status
Adequate energy and fluid intake and sustained hydration should be reinforced
at each visit9,10
(Evaluated every 1-4 weeks)
Included in ASPEN Consensus Recommendations for monitoring during parenteral nutrition
Eliminating PS requirements is the ultimate goal for all patients with SBS, however reducing the hours per day or days per week on PS can provide substantial benefits.16
Initiate PS Weaning for your appropriate patients with this comprehensive guide

Lisa | 49 years

Sonia | 42 years
History of illness
Lisa’s experience starting GATTEX
Lisa’s experience weaning with GATTEX
*Reductions followed by safety evaluation after 1 week, including 48-hour urine collection and clinical evaluation: signs and symptoms of dehydration, weight changes, review of recorded oral fluid intake, blood samples (hematocrit, creatinine, blood urea nitrogen), and urine sodium.
History of illness
Sonia’s experience starting GATTEX
Sonia’s experience weaning with GATTEX
*Reductions followed by safety evaluation after 1 week, including 48-hour urine collection and clinical evaluation: signs and symptoms of dehydration, weight changes, review of recorded oral fluid intake, blood samples (hematocrit, creatinine, blood urea nitrogen), and urine sodium.

GATTEX® (teduglutide) for injection is indicated for the treatment of adults and pediatric patients 1 year of age and older with short bowel syndrome (SBS) who are dependent on parenteral support.
Warnings and Precautions
GATTEX has been associated with acceleration of neoplastic growth, intestinal obstruction, biliary and pancreatic disease, fluid imbalance and fluid overload, and increased absorption of concomitant oral medication. Click here for additional Safety Information.
INDICATION
GATTEX® (teduglutide) for injection is indicated
for the treatment of adults and pediatric patients 1 year of age and older with short bowel syndrome (SBS) who
are dependent on parenteral support.
IMPORTANT SAFETY INFORMATION
Warnings and Precautions
Acceleration of Neoplastic growth
Intestinal polyps were identified during clinical trials. Postmarketing cases of colorectal, gastric, and small intestinal (duodenum, ileum, and jejunum) polyps have been reported. There is a risk for acceleration of neoplastic growth. In adults, within 6 months prior to starting treatment with GATTEX, perform colonoscopy and an upper gastrointestinal (GI) endoscopy with removal of polyps. A follow-up colonoscopy and upper GI endoscopy (or alternate imaging) is recommended at the end of 1 year of GATTEX. Subsequent colonoscopies and upper GI endoscopies (or alternate imaging) should be performed every 5 years or more often as needed. If a polyp is found, adherence to current polyp follow-up guidelines is recommended.
In pediatric patients, perform fecal occult blood testing within 6 months prior to initiating treatment with GATTEX. If there is new or unexplained blood in the stool, perform colonoscopy/sigmoidoscopy and an upper GI endoscopy. Perform subsequent fecal occult blood testing annually in pediatric patients while they are receiving GATTEX, followed by colonoscopy/sigmoidoscopy and an upper GI endoscopy if there is new or unexplained blood in the stool. Colonoscopy/sigmoidoscopy is recommended for all pediatric patients after 1 year of treatment and at least every 5 years thereafter while on continuous treatment with GATTEX. Consider upper GI endoscopy (or alternate imaging) during treatment with GATTEX.
In adult and pediatric patients who develop active gastrointestinal malignancy (GI tract, hepatobiliary, pancreatic), discontinue GATTEX. The clinical decision to continue GATTEX in patients with non-gastrointestinal malignancy should be made based on benefit-risk considerations.
Intestinal obstruction
Intestinal obstruction has been reported in clinical trials
and postmarketing. In patients who develop intestinal or stomal obstruction, GATTEX should be temporarily
discontinued pending further clinical evaluation and management.
Biliary and pancreatic disease
Cholecystitis, cholangitis, cholelithiasis, and
pancreatitis have been reported in clinical trials and postmarketing. Laboratory assessment (bilirubin,
alkaline phosphatase, lipase, amylase) should be obtained within 6 months prior to starting GATTEX. Subsequent
laboratory tests should be done every 6 months or more often as needed. If clinically meaningful changes are
seen, further evaluation is recommended including imaging, and continued treatment with GATTEX should be
reassessed.
Fluid imbalance and fluid overload
Fluid overload and congestive heart failure
have been observed in clinical trials. If fluid overload occurs, especially in patients with underlying
cardiovascular disease, parenteral support should be adjusted and GATTEX treatment reassessed. If significant
cardiac deterioration develops while on GATTEX, continued GATTEX treatment should be reassessed.
Discontinuation of treatment with GATTEX may also result in fluid and electrolyte imbalance. Fluid and electrolyte status should be monitored in patients who discontinue treatment with GATTEX.
Increased absorption of concomitant oral medication
In clinical trials, one
patient receiving prazepam concomitantly with GATTEX experienced dramatic deterioration in mental status
progressing to coma during first week of GATTEX therapy. Patients receiving concomitant oral drugs requiring
titration or with a narrow therapeutic index should be monitored for adverse reactions due to potential
increased absorption of the concomitant drug. The concomitant drug may require a reduction in dosage.
Adverse Reactions
The most common adverse reactions (≥ 10%) with GATTEX are
abdominal pain, nausea, upper respiratory tract infection, abdominal distension, injection site reaction,
vomiting, fluid overload, and hypersensitivity.
Use in Specific Populations
Breastfeeding is not recommended during
treatment with GATTEX.
Please click here for full Prescribing Information or Información de prescripción en español.
SCROLL FOR FULL PRESCRIBING INFORMATION
These highlights do not include all the information needed to use GATTEX safely and effectively. See full prescribing information for GATTEX.
GATTEX® (teduglutide) for injection, for subcutaneous use
Initial U.S. Approval: 2012
Dosage and Administration
| Important Administration Information (2.1) | 09/2024 |
| Monitoring to Assess Safety (2.4) | 09/2024 |
| Acceleration of Neoplastic Growth (5.1) | 09/2024 |
GATTEX® is a glucagon-like peptide-2 (GLP-2) analog indicated for the treatment of adults and pediatric patients 1 year of age and older with Short Bowel Syndrome (SBS) who are dependent on parenteral support. (1)
Important Administration Information
GATTEX is for adult self-administration or caregiver administration. Self-administration in pediatric patients has not been tested. Use of the GATTEX 5 mg kit is not recommended in pediatric patients weighing less than 10 kg. (2.1)
Evaluation Testing within 6 Months Prior to Starting GATTEX
Dosage and Administration
Dosage Adjustment for Renal Impairment
Discontinuation
Preparation
For injection: 5 mg teduglutide in a single-dose vial supplied with 0.5 mL Sterile Water for Injection in a single-dose prefilled syringe. (3)
None. (4)
Most common adverse reactions (≥10%) are: abdominal pain, nausea, upper respiratory tract infection, abdominal distension, injection site reaction, vomiting, fluid overload, and hypersensitivity. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Takeda Pharmaceuticals U.S.A., Inc. at 1-877-TAKEDA-7 (1-877-825-3327) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Lactation: Breastfeeding not recommended. (8.2)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 9/2024
*Sections or subsections omitted from the full prescribing information are not listed.
GATTEX® is indicated for the treatment of adults and pediatric patients 1 year of age and older with Short Bowel Syndrome (SBS) who are dependent on parenteral support.
2.1 Important Administration Information
GATTEX is for adult self-administration or caregiver administration. Self-administration in pediatric patients has not been tested.
Use of the GATTEX 5 mg kit is not recommended in pediatric patients weighing less than 10 kg.
Evaluation and testing prior to starting treatment with GATTEX:
Within 6 months prior to treatment:
Adult patients
Pediatric patients
2.2 Recommended Dosage and Administration for Adults and Pediatric Patients 1 Year of Age and Older
GATTEX is for subcutaneous injection only. Not for intravenous or intramuscular administration.
The recommended dosage of GATTEX is 0.05 mg/kg once daily administered by subcutaneous injection.
If a dose is missed, that dose should be taken as soon as possible on that day. Do not take 2 doses on the same day.
Alternation of sites for subcutaneous injection is recommended, and can include the thighs, upper arms, and the abdomen.
2.3 Dosage Adjustment for Renal Impairment
The recommended dosage in adult and pediatric patients with moderate and severe renal impairment and end-stage renal disease (estimated glomerular filtration rate (eGFR) less than 60 mL/min/1.73 m2) is 0.025 mg/kg once daily [see Use in Specific Populations (8.6)].
2.4 Monitoring to Assess Safety
Colonoscopy and Upper GI Endoscopy in Adults
Colonoscopy and Upper GI Endoscopy in Pediatric Patients
Laboratory Testing
Laboratory assessments are recommended every 6 months. If any clinically meaningful elevation is seen, further diagnostic workup is recommended as clinically indicated (i.e., imaging of the biliary tract, liver, or pancreas) [see Warnings and Precautions (5.1), (5.3)].
2.5 Discontinuation of Treatment
Discontinuation of treatment with GATTEX may result in fluid and electrolyte imbalance. Monitor fluid and electrolyte status in patients who discontinue GATTEX treatment [see Warnings and Precautions (5.4)].
2.6 Preparation Instructions
Storage of the reconstituted solution
For Injection: 5 mg teduglutide as a white lyophilized powder for reconstitution in a single-dose vial supplied with 0.5 mL Sterile Water for Injection in a single-dose prefilled syringe.
None.
5.1 Acceleration of Neoplastic Growth
Based on the pharmacologic activity and tumor findings in the rat and mouse carcinogenicity studies, GATTEX has the potential to cause hyperplastic changes including neoplasia [see Clinical Pharmacology (12.1), Nonclinical Toxicology (13.1)]. In patients at increased risk for malignancy, the clinical decision to use GATTEX should be considered only if the benefits outweigh the risks. In patients who develop active gastrointestinal malignancy (GI tract, hepatobiliary, pancreatic) while on GATTEX, discontinue GATTEX treatment. In patients who develop active non-gastrointestinal malignancy while on GATTEX, the clinical decision to continue GATTEX should be made based on benefit-risk considerations.
Gastrointestinal Polyps
Intestinal polyps were identified during the clinical studies. Postmarketing cases of colorectal, gastric, and small intestinal (duodenum, ileum, and jejunum) polyps have been reported postmarketing [see Adverse Reactions (6.1, 6.2)].
Adult Patients
Pediatric Patients
5.2 Intestinal Obstruction
Intestinal obstruction has been reported in clinical studies [see Adverse Reactions (6.1)] and postmarketing. In patients who develop intestinal or stomal obstruction, temporarily discontinue GATTEX while the patient is clinically managed. GATTEX may be restarted when the obstructive presentation resolves, if clinically indicated.
5.3 Biliary and Pancreatic Disease
Gallbladder and Biliary Tract Disease
Cholecystitis, cholangitis, and cholelithiasis have been reported in clinical studies [see Adverse Reactions (6.1)] and postmarketing. For identification of the onset or worsening of gallbladder/biliary disease, obtain laboratory assessment of bilirubin and alkaline phosphatase within 6 months prior to starting GATTEX, and at least every 6 months while on GATTEX; or more frequently if needed. If clinically meaningful changes are seen, further evaluation including imaging of the gallbladder and/or biliary tract is recommended; and reassess the need for continued GATTEX treatment.
Pancreatic Disease
Pancreatitis has been reported in clinical studies [see Adverse Reactions (6.1)]. For identification of onset or worsening of pancreatic disease, obtain laboratory assessments of lipase and amylase within 6 months prior to starting GATTEX, and at least every 6 months while on GATTEX; or more frequently if needed. If clinically meaningful changes are seen, further evaluation such as imaging of the pancreas is recommended; and reassess the need for continued GATTEX treatment.
5.4 Fluid Imbalance and Fluid Overload
Fluid Overload
Fluid overload and congestive heart failure have been observed in clinical studies, which were deemed to be related to enhanced fluid absorption associated with GATTEX [see Adverse Reactions (6.1)]. If fluid overload occurs, adjust parenteral support and reassess GATTEX treatment, especially in patients with underlying cardiovascular disease. If significant cardiac deterioration develops while on GATTEX, reassess the need for continued GATTEX treatment.
Fluid and Electrolyte Imbalance
Discontinuation of treatment with GATTEX may also result in fluid and electrolyte imbalance. Monitor fluid and electrolyte status in patients who discontinue treatment with GATTEX [see Dosage and Administration (2.5)].
5.5 Increased Absorption of Concomitant Oral Medication
In the adult placebo-controlled studies, an analysis of episodes of cognition and attention disturbances was performed for patients on benzodiazepines. One patient receiving prazepam concomitantly with GATTEX 0.05 mg/kg once daily experienced a dramatic deterioration in mental status progressing to coma during the first week of GATTEX therapy. The patient was admitted to the ICU and the prazepam blood concentration was >300 mcg/L. GATTEX and prazepam were discontinued, and coma resolved 5 days later.
Monitor patients receiving concomitant oral drugs requiring titration or with a narrow therapeutic index, for adverse reactions due to potential increased absorption of the concomitant drug. The concomitant drug may require a reduction in dosage [see Drug Interactions (7.1)].
The following serious adverse reactions are described elsewhere in the labeling:
6.1 Clinical Trials Experience
Adults
Because clinical trials are conducted under widely varying conditions, the adverse reaction rates observed cannot be directly compared to rates in other clinical trials and may not reflect the rates observed in clinical practice.
The rates of adverse reactions in 136 adult patients with SBS participating in two randomized, placebo-controlled, 24-week, double-blind clinical studies (Study 1 and Study 3) are summarized in Table 1. Only those reactions with a rate of at least 5% in the GATTEX group, and greater than placebo group, are summarized in Table 1.
Table 1: Common Adverse Reactions* in Adult Patients with SBS in Placebo-Controlled Studies: Studies 1 and 3
| Adverse Reaction | Placebo (N=59) (%) |
GATTEX 0.05 mg/kg Once Daily (N=77) (%) |
|---|---|---|
| Abdominal pain1 | 22 | 30 |
| Nausea | 20 | 23 |
| Upper respiratory tract infection2 | 12 | 21 |
| Abdominal distension | 2 | 20 |
| Injection site reaction3 | 12 | 13 |
| Vomiting | 10 | 12 |
| Fluid overload4 | 7 | 12 |
| Hypersensitivity5 | 7 | 10 |
| Flatulence | 7 | 9 |
| Decreased appetite | 3 | 7 |
| Influenza6 | 2 | 7 |
| Skin hemorrhage7 | 2 | 5 |
| Cough | 0 | 5 |
| Sleep disturbances8 | 0 | 5 |
Reported at a rate of at least 5% in the GATTEX group, and greater than the placebo group.
Includes: Abdominal pain, upper abdominal pain, lower abdominal pain
Includes: Upper respiratory tract infection, nasopharyngitis, pharyngitis, sinusitis, laryngitis, rhinitis, viral upper respiratory tract infection
Includes: Injection site hematoma, injection site erythema, injection site pain, injection site swelling, injection site hemorrhage, injection site discoloration, injection site reaction, injection site rash
Includes: Fluid overload, peripheral edema, edema, generalized edema, fluid retention and jugular vein distension
Includes: Erythema, rash, dermatitis allergic, pruritus, rash macular, drug eruption, eyelid edema, flushing
Includes: Influenza, influenza-like illness
Includes: Hematoma, abdominal wall hematoma, post procedural hematoma, umbilical hematoma, blood blister
Includes: Insomnia (3 patients) and hypersomnia (1 patient)
Adverse Reactions in the Subset of Patients with a Stoma
Among the 53 patients with a stoma in the placebo-controlled studies (Study 1 and Study 3), the percentage of patients with gastrointestinal stoma complication was 42% (13/31) for patients receiving GATTEX 0.05 mg/kg/day and 14% (3/22) for patients receiving placebo.
Pediatric Patients 1 Year to Less Than 17 Years of Age
In two clinical studies of 24-week and 12-week duration, 41 pediatric patients aged 1 year to less than 17 years were treated with GATTEX 0.05 mg/kg/day [see Use in Specific Populations (8.4), Clinical Studies (14.2)]. Overall, the safety profile of GATTEX was similar to that in adults. In the long-term extension studies with mean exposure of 41 weeks, no new safety signals were identified.
Less Common Adverse Reactions
Adverse Reactions of Special Interest
Malignancy
Three patients were diagnosed with malignancy in the SBS clinical studies in adults, all of whom were male and had received GATTEX 0.05 mg/kg/day in Study 2. One patient had a history of abdominal radiation for Hodgkin’s disease two decades prior to receiving GATTEX and prior liver lesion on CT scan, and was diagnosed with metastatic adenocarcinoma of unconfirmed origin after 11 months of exposure to GATTEX. Two patients had extensive smoking histories and were diagnosed with lung cancers (squamous and non-small cell) after 12 months and 3 months of GATTEX exposure, respectively [see Warnings and Precautions (5.1)].
Intestinal Polyps
In the adult clinical studies, 14 patients with SBS were diagnosed with polyps of the GI tract after initiation of study treatment. In the SBS placebo-controlled studies, 1/59 (2%) of patients on placebo and 1/109 (1%) of patients on GATTEX 0.05 mg/kg/day were diagnosed with intestinal polyps (inflammatory stomal and hyperplastic sigmoidal after 3 and 5 months, respectively). The remaining 12 polyp cases occurred in the extension studies — 2 colorectal villous adenomas (onset at 6 and 7 months in GATTEX 0.1 mg/kg/day (twice the recommended dose) and 0.05 mg/kg/day dose groups, respectively), 2 hyperplastic polyps (onset 6 months in GATTEX 0.1 mg/kg/day dose group and 24 months in GATTEX 0.05 mg/kg/day dose group), 4 colorectal tubular adenomas (onset between 24 and 29 months in GATTEX 0.05 mg/kg/day dose group), 1 serrated adenoma (onset at 24 months in GATTEX 0.05 mg/kg/day dose group), 1 colorectal polyp biopsy not done (onset at 24 months in GATTEX 0.05 mg/kg/day dose group), 1 rectal inflammatory polyp (onset at 10 months in the GATTEX 0.05 mg/kg/day dose group, and 1 small duodenal polyp (onset at 3 months in GATTEX 0.05 mg/kg/day dose group) [see Warnings and Precautions (5.1)].
In the pediatric clinical studies (up to 69 weeks of exposure), there was one case of cecal polyp that was not biopsied and was not seen on repeat colonoscopy.
Gastrointestinal Obstruction
Overall, in the adult clinical studies, 12 patients with SBS experienced one or more episodes of intestinal obstruction/stenosis: 6 in SBS placebo-controlled studies and 6 in the extension studies. The 6 patients in the placebo-controlled studies were all on GATTEX: 3/77 (4%) on GATTEX 0.05 mg/kg/day and 3/32 (9%) on GATTEX 0.1 mg/kg/day (twice the recommended dose). No cases of intestinal obstruction occurred in the placebo group. Onset ranged from 1 day to 6 months. In the adult extension studies, 6 additional patients (all on GATTEX 0.05 mg/kg/day) were diagnosed with intestinal obstruction/stenosis with onsets ranging from 6 days to 19 months. Two of the 6 patients from the placebo-controlled studies experienced recurrence of obstruction in the extension studies. Of all 8 patients with an episode of intestinal obstruction/stenosis in these extension studies, 2 patients required endoscopic dilation and 1 required surgical intervention) [see Warnings and Precautions (5.2)].
In the pediatric clinical studies (up to 69 weeks of exposure), there was 1 serious adverse reaction of obstruction. Teduglutide was temporarily withheld, obstruction resolved without additional intervention, and there was no recurrence once teduglutide was restarted.
Gallbladder, Biliary and Pancreatic Disease
For gallbladder and biliary disease in the adult placebo-controlled studies, 3 patients with SBS were diagnosed with cholecystitis, all of whom had a prior history of gallbladder disease and were in the GATTEX 0.05 mg/kg/day dose group. No cases were reported in the placebo group. One of these 3 cases had gallbladder perforation and underwent cholecystectomy the next day. The remaining 2 cases underwent elective cholecystectomy at a later date. In the adult extension studies, 4 patients had an episode of acute cholecystitis; 3 patients had new-onset cholelithiasis; and 1 patient experienced cholestasis secondary to an obstructed biliary stent. For pancreatic disease in the adult placebo-controlled studies, 1 patient (GATTEX 0.05 mg/kg/day dose group) had a pancreatic pseudocyst diagnosed after 4 months of GATTEX. In the adult extension studies, 1 patient was diagnosed with chronic pancreatitis; and 1 patient was diagnosed with acute pancreatitis) [see Warnings and Precautions (5.3)].
Fluid Overload
In the adult placebo-controlled studies, peripheral edema was reported in 2/59 (3%) of patients on placebo and 8/77 (10%) patients on GATTEX; fluid overload was reported in 1/77 (1%) patient in the GATTEX group; no cases of fluid overload were seen in the placebo arm. There were 2 cases of congestive heart failure (CHF, 3%) in the GATTEX arm, 1 of which was reported as a serious adverse event and the other as non-serious. The serious case had onset at 6 months and was possibly associated with previously undiagnosed hypothyroidism and/or cardiac dysfunction [see Warnings and Precautions (5.4)].
Other Less Common Adverse Reactions
Reported in less than 5% of patients treated with GATTEX:
Gastrointestinal disorders: Colonic stenosis, Pancreatic duct stenosis, Small intestinal stenosis
Respiratory, thoracic and mediastinal disorders: Dyspnea
6.2 Postmarketing Experience
The following adverse reactions have been identified during post approval use of teduglutide. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Gastrointestinal Neoplasia: colorectal polyps, gastric polyps, small intestinal polyps (duodenum, ileum, and jejunum)
7.1 Potential for Increased Absorption of Oral Medications
Based upon the pharmacodynamic effect of GATTEX, there is a potential for increased absorption of concomitant oral medications. Altered mental status has been observed in patients taking GATTEX and benzodiazepines in the adult clinical studies [see Warnings and Precautions (5.5)].
Monitor patients on concomitant oral drugs requiring titration or with a narrow therapeutic index for adverse reactions related to the concomitant drug while on GATTEX. The concomitant drug may require a reduction in dosage.
8.1 Pregnancy
Risk Summary
Available data from case reports with GATTEX use in pregnant women have not identified a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes. Pregnant women with short bowel syndrome are at risk for malnutrition, which is associated with adverse maternal and fetal outcomes (see Clinical Considerations). In animal reproduction studies, no effects on embryo-fetal development were observed with the subcutaneous administration of teduglutide to pregnant rats and rabbits during organogenesis at exposures up to 686 and 657 times, respectively, the clinical exposure at the recommended human dose (based on AUC) (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Clinical Considerations
Disease-associated maternal and/or embryo/fetal risk
Pregnant women with short bowel syndrome are at risk for malnutrition. Severe malnutrition in pregnant women is associated with preterm delivery, low birth weight, intrauterine growth restriction, congenital malformations and perinatal mortality.
Data
Animal Data
Reproduction studies have been performed in pregnant rats at subcutaneous doses of teduglutide up to 25 mg/kg twice daily (50 mg/kg/day) (about 686 times the clinical exposure (AUC) at the recommended daily human dose of 0.05 mg/kg) and in pregnant rabbits at subcutaneous doses up to 25 mg/kg twice daily (50 mg/kg/day) (about 657 times the clinical exposure (AUC) at the recommended daily human dose of 0.05 mg/kg) during the period of organogenesis. These studies did not reveal any evidence of impaired fertility or harm to the fetus due to teduglutide. In a pre- and postnatal development study in rats (gestation day 7 to lactation day 20), teduglutide did not show any significant adverse effects on pre- and postnatal development at doses up to 25 mg/kg twice daily (50 mg/kg/day) (about 343 times the clinical exposure (AUC) at the recommended daily human dose of 0.05 mg/kg).
8.2 Lactation
Risk Summary
There is no information regarding the presence of GATTEX in human milk, the effects of GATTEX on the breastfed infant, or the effects of GATTEX on milk production. Teduglutide is present in the milk of lactating rats (see Data). Systemic exposure of teduglutide to a breastfed infant is expected to be low. However, because of the potential for serious adverse reactions in a breastfed infant, including tumorigenicity [see Nonclinical Toxicology (13.1)], advise patients that breastfeeding is not recommended during treatment with GATTEX.
Data
In a milk excretion study in the rat, a single subcutaneous dose of 25 mg/kg of teduglutide (81 times the recommended daily human dose of 0.05 mg/kg based on body surface area) was administered to lactating female rats at Day 12 postpartum. The maximum concentration of teduglutide in the milk corresponded to 0.9% and 2.9% of the plasma concentration at 1.5 and 4 hours after dosing, respectively.
8.4 Pediatric Use
The safety and effectiveness in pediatric patients less than 1 year of age have not been established.
The safety and effectiveness of GATTEX have been established in pediatric patients 1 year to less than 17 years of age who are dependent on parenteral support for the treatment of SBS. Use of GATTEX in this population is supported by evidence from adequate and well-controlled studies in adults, with additional efficacy, safety, pharmacokinetic and pharmacodynamic data in pediatric patients 1 year to less than 17 years of age [see Dosage and Administration (2), Adverse Reactions (6.1), Clinical Pharmacology (12.3), Clinical Studies (14.2)]. These data were derived from two studies of 24-week (Study 5) and 12-week (NCT01952080) duration in which 41 pediatric patients were treated with GATTEX in the following groups: 1 infant (1 year to less than 2 years), 37 children (2 years to less than 12 years) and 3 adolescents (12 years to less than 17 years).
In these 2 studies and the corresponding extension studies (Study 6 and NCT02949362), 29 pediatric patients were administered GATTEX prospectively for up to 94 weeks [see Clinical Studies (14.2)]. Adverse reactions in pediatric patients were similar to those seen in adults [see Adverse Reactions (6.1)].
Juvenile Animal Toxicity Data
In a juvenile toxicity study, teduglutide was administered to juvenile minipigs at subcutaneous doses of 0.5, 2.5 and 12.5 mg/kg twice daily (1, 5, and 25 mg/kg/day) from post-natal day 7 and continuing for 90 days). Exposures (AUC) at these doses were at least 12-, 25-, and 170-fold the pediatric clinical exposure for ages 1 year to 11 years at 0.05 mg/kg, respectively, and 10-, 21-, and 141-fold the pediatric clinical exposure for ages 12 years to 17 years at 0.05 mg/kg, respectively.
In juvenile minipigs, subcutaneous teduglutide caused intestinotrophic effects, gall bladder mucosal hyperplasia, bile duct mucosal hyperplasia, and injection site reactions, similar to those observed in adult animals.
8.5 Geriatric Use
Of the 134 patients with SBS that were treated with GATTEX at the recommended dosage of 0.05 mg/kg/day in the clinical studies, 19 patients were 65 years or older while 5 patients were 75 years of age or older. No overall differences in safety or efficacy were observed between these patients and younger patients, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out [see Clinical Pharmacology (12.3)].
8.6 Renal Impairment
In adult subjects with moderate to severe renal impairment or end-stage renal disease (ESRD) (creatinine clearance <60 mL/min), the exposure to teduglutide increased with the degree of renal impairment [see Clinical Pharmacology (12.3)]. Reduce the dosage of GATTEX by half in both pediatric and adult patients with eGFR less than 60 mL/min/1.73 m2 [see Dosage and Administration (2.3)].
8.7 Hepatic Impairment
GATTEX has not been studied in patients with severe hepatic impairment (Child-Pugh grade C). No dosage adjustment is recommended for patients with mild and moderate hepatic impairment (Child-Pugh grade A and B) [see Clinical Pharmacology (12.3)].
The maximum dose of GATTEX studied during clinical development was 80 mg/day for 8 days. No unexpected systemic adverse reactions were seen. In the event of overdose, the patient should be carefully monitored by the medical professional.
The active ingredient in GATTEX (teduglutide) for injection is teduglutide, which is a 33 amino acid glucagon-like peptide-2 (GLP-2) analog manufactured using a strain of Escherichia coli modified by recombinant DNA technology. The chemical composition of teduglutide is L-histidyl-L-glycyl-L-aspartyl-L-glycyl-L-seryl-L-phenylalanyl-L-seryl-L-aspartyl-L-glutamyl-L-methionyl-L-asparaginyl-L-threonyl-L-isoleucyl-L-leucyl-L-aspartyl-L-asparaginyl-L-leucyl-L-alanyl-L-alanyl-L-arginyl-L-aspartyl-L-phenylalanyl-L-isoleucyl-L-asparaginyl-L-tryptophanyl-L-leucyl-L-isoleucyl-L-glutaminyl-L-threonyl-L-lysyl-L-isoleucyl-L-threonyl-L-aspartic acid. The structural formula is:
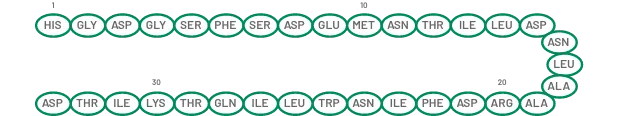
Figure 1: Structural formula of teduglutide
Teduglutide has a molecular weight of 3752 Daltons. Teduglutide drug substance is a clear, colorless to light-straw–colored liquid.
Each single-dose vial of GATTEX contains 5 mg of teduglutide as a white lyophilized powder for reconstitution and administration by subcutaneous injection. In addition to the active pharmaceutical ingredient (teduglutide), each vial of GATTEX contains 3.434 mg dibasic sodium phosphate heptahydrate, 3.88 mg L-histidine, 15 mg mannitol, and 0.644 mg monobasic sodium phosphate monohydrate as excipients. No preservatives are present.
At the time of administration, the lyophilized powder is reconstituted with 0.5 mL of Sterile Water for Injection, which is provided in a single-dose prefilled syringe. A 10 mg/mL sterile solution is obtained after reconstitution. Up to 0.38 mL of the reconstituted solution which contains 3.8 mg of teduglutide can be withdrawn for subcutaneous injection upon reconstitution.
12.1 Mechanism of Action
Teduglutide is an analog of naturally occurring human glucagon-like peptide-2 (GLP-2), a peptide secreted by L-cells of the distal intestine. GLP-2 is known to increase intestinal and portal blood flow and inhibit gastric acid secretion. Teduglutide binds to the glucagon-like peptide-2 receptors located in intestinal subpopulations of enteroendocrine cells, subepithelial myofibroblasts and enteric neurons of the submucosal and myenteric plexus. Activation of these receptors results in the local release of multiple mediators including insulin-like growth factor (IGF)-1, nitric oxide and keratinocyte growth factor (KGF).
12.2 Pharmacodynamics
Intestinal Fluid Absorption
The ability of GATTEX to improve intestinal absorption was studied in 17 adult subjects with Short Bowel Syndrome (N=2-3 per dose group) using daily doses of 0.03, 0.1, 0.15 mg/kg (doses ranging from 0.6 to 3 times the recommended dose) in a 21-day, open-label, multicenter, dose-ranging study. All subcutaneous (abdomen) doses studied, except 0.03 mg/kg once daily, resulted in enhanced gastrointestinal fluid (wet weight) absorption of approximately 750 to 1000 mL/day, and increased villus height and crypt depth of the intestinal mucosa.
Cardiac Electrophysiology
At a dose 5 times the recommended dose, GATTEX did not prolong the QT interval to any clinically relevant extent.
12.3 Pharmacokinetics
Absorption
In healthy subjects, GATTEX administered subcutaneously had an absolute bioavailability of 88% and reached maximum plasma teduglutide concentrations at 3 to 5 hours after administration. Following a 0.05 mg/kg subcutaneous dose in SBS subjects, the median peak teduglutide concentration (Cmax) was 36 ng/mL and the median area under the curve at steady state (AUCtau) was 0.15 μg•hr/mL. No accumulation of teduglutide was observed following repeated subcutaneous administrations.
The Cmax and AUC of teduglutide was dose proportional over the dose range of 0.05 to 0.4 mg/kg (up to 8 times the recommended dose of GATTEX).
Distribution
In healthy subjects, teduglutide has a volume of distribution (103 mL/kg) similar to blood volume.
Elimination
Metabolism
The metabolic pathway of teduglutide was not investigated in humans. However, teduglutide is expected to be degraded into small peptides and amino acids via catabolic pathways, similar to the catabolism of endogenous GLP-2.
Excretion
In healthy subjects, teduglutide plasma clearance was approximately 123 mL/hr/kg which is similar to the GFR suggesting that teduglutide is primarily cleared by the kidney. Teduglutide has a mean terminal half-life (t1/2) of approximately 2 hours in healthy subjects and 1.3 hours in SBS subjects.
Use in Specific Populations
Geriatric Patients
No differences were observed between healthy subjects younger than 65 years and those older than 65 years. Experience in subjects 75 years and above is limited.
Pediatric Patients
Following subcutaneous administration, similar steady state Cmax of teduglutide across age groups was demonstrated by population pharmacokinetics modeling (see Table 2). However, lower AUC was seen in pediatric patients 1 to 17 years of age as compared with adults and increases with increasing age.
Table 2: Teduglutide Pharmacokinetic Parameters Following Subcutaneous Dosing of GATTEX 0.05 mg/kg by Age Groups
| Parameters (Mean ± SD) | ||||
|---|---|---|---|---|
| Age | Cmax, ss (ng/mL) | AUCss (ng•h/mL) | CL/F (L/h) | t1/2 (h) |
| 12 to 17 years (n=3) | 29.7 ± 8.4 | 154 ± 17.6 | 13.0 ± 2.3 | 1.0 ± 0.01 |
| 1 to 11 years (n=37) | 33.5 ± 11.5 | 128 ± 56.7 | 7.45 ± 2.1 | 0.7 ± 0.2 |
Male and Female Patients
No clinically relevant gender differences were observed.
Patients with Renal Impairment
In adult subjects with moderate to severe renal impairment or end stage renal disease (ESRD) (creatinine clearance <60 mL/min), the Cmax and AUC0-inf of teduglutide increased with the degree of renal impairment following a single subcutaneous dose of 10 mg GATTEX. Teduglutide exposure increased by a factor of 1.6, 1.4, and 2.1 (Cmax) and 1.5, 1.7, and 2.6 (AUC0-inf) in subjects with moderate, severe renal impairment and ESRD, respectively, compared to healthy subjects [see Dosage and Administration (2.3), Use in Specific Populations (8.6)].
Patients with Hepatic Impairment
Subjects with moderate hepatic impairment (Child-Pugh Class B) had an approximately 10 to 15% lower teduglutide Cmax and AUC compared to healthy matched control subjects after a single subcutaneous dose of 20 mg GATTEX. This reduction in teduglutide exposure is not thought to be clinically meaningful. GATTEX was not studied in subjects with severe hepatic impairment (Child-Pugh Class C).
Drug Interaction Studies
Clinical interaction studies were not performed. No inhibition or induction of the cytochrome P450 enzyme system has been observed based on in vitro studies although the relevance of in vitro studies to an in vivo setting is unknown.
12.6 Immunogenicity
The observed incidence of anti-drug antibodies is highly dependent on the sensitivity and specificity of the assay. Differences in assay methods preclude meaningful comparisons of the incidence of anti-drug antibodies in the studies described below with the incidence of anti-drug antibodies in other studies, including those of GATTEX or of other teduglutide products.
Adults
Based on integrated data from two studies in adults with SBS (a 6‑month randomized placebo‑controlled study, followed by a 24‑month open‑label study), the development of anti‑teduglutide antibodies in patients who received subcutaneous administration of 0.05 mg/kg GATTEX once daily was 3% (2/60) at Month 3, 17% (13/77) at Month 6, 24% (16/67) at Month 12, 33% (11/33) at Month 24, and 48% (14/29) at Month 30. Anti-teduglutide antibodies were cross-reactive to native glucagon-like peptide (GLP‑2) in 5 of the 6 patients (83%) who had anti-teduglutide antibodies and were tested for cross‑reactivity. In the same two studies, a total of 36 patients were tested for neutralizing antibodies: one patient developed borderline positive neutralizing antibody responses at month 24 of the extension study. The antibody formation has not been associated with clinically relevant safety findings, reduced efficacy or changed pharmacokinetics of GATTEX.
Pediatric Patients 1 Year to Less than 17 Years of Age
In pediatric patients who received subcutaneous administration of 0.05 mg/kg GATTEX once daily for 24 weeks, the rate of anti‑teduglutide antibody formation at Month 6 was 19% (5/26) and was similar to the rate of antibody formation in adult patients (17%). Of the 5 pediatric patients who had developed antibodies to teduglutide at Month 6, 2 patients had neutralizing antibodies. However, with the longer duration of treatment, the rate of anti‑teduglutide formation at Month 12 was higher in pediatric patients with 54% (14/26), compared to that of adults (24%). Of the 14 pediatric patients who had developed antibodies to teduglutide at Month 12, 1 patient had neutralizing antibodies.
Among the small number of pediatric patients who developed anti‑teduglutide antibodies, no association with adverse events or lack of efficacy was observed.
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenic potential of GATTEX was assessed in 2-year subcutaneous carcinogenicity studies in rats and mice. In a 2-year carcinogenicity study in Wistar Han rats at subcutaneous doses of 3, 10, and 35 mg/kg/day (about 15, 41, and 199 times the exposures [AUC] achieved at the recommended daily human dose of 0.05 mg/kg, respectively), teduglutide caused statistically significant increases in the incidences of adenomas in the bile duct and jejunum of male rats. In a 2-year carcinogenicity study in Crl:CD1(ICR) mice at subcutaneous doses of 1, 3.5, and 12.5 mg/kg/day (about 32, 66, and 244 times the exposures [AUC] achieved at the recommended daily human dose of 0.05 mg/kg, respectively), teduglutide caused a significant increase in papillary adenomas in the gall bladder; it also caused adenocarcinomas in the jejunum in male mice at the high dose of 12.5 mg/kg/day.
Teduglutide was negative in the Ames test, chromosomal aberration test in Chinese hamster ovary cells, and in vivo mouse micronucleus assay.
Teduglutide at subcutaneous doses up to 25 mg/kg twice daily (50 mg/kg/day or at least 202 times the clinical exposure (AUC) at the recommended daily human dose of 0.05 mg/kg) was found to have no adverse effect on fertility and reproductive performance of male and female rats.
14.1 Treatment of SBS in Adults
Study 1 (Placebo-controlled) and Study 2 (Open-label Extension of Study 1)
Study 1 (CL0600-020, NCT00798967)
The efficacy, safety, and tolerability of GATTEX was evaluated in a randomized, double-blind, placebo-controlled, parallel-group, multi-national, multicenter clinical study (Study 1) in adults with SBS who were dependent on parenteral nutrition/intravenous (PN/I.V.) support for at least 12 months and required PN at least 3 times per week. For 8 weeks (or less) prior to randomization, investigators optimized the PN/I.V. volume of all patients. Optimization was followed by a 4-week to 8-week period of fluid stabilization. Patients then were randomized (1:1) to placebo (n=43) or GATTEX 0.05 mg/kg/day (n=43). Study treatment was administered subcutaneously once daily for 24 weeks. PN/I.V. volume adjustments (up to 30% decrease) and clinical assessments were made at 2, 4, 8, 12, 20, and 24 weeks.
The primary efficacy endpoint was based on a clinical response, defined as a patient achieving at least 20% reduction in weekly PN/I.V. volume from Baseline (immediately before randomization) to both Weeks 20 and 24.
The mean age of patients was 50 years. Mean duration of PN/I.V. dependency prior to enrollment was 6 years (range 1 to 26 years). The most common reasons for intestinal resection leading to SBS were vascular disease (34%, 29/85), Crohn’s Disease (21%, 18/85), and “other” (21%, 18/85). Stoma was present in 45% (38/85) of patients, and the most common type was jejunostomy/ileostomy (82%, 31/38). The mean length of remaining small intestine was 77.3±64.4 cm (range: 5 to 343 cm). The colon was not in continuity in 44% (37/85) patients. At baseline, the mean (± SD) prescribed days per week for PN/I.V. infusion was 5.73 (±1.59) days.
The percentages of treatment group responders were compared in the intent-to-treat population of this study which was defined as all randomized patients. Sixty-three percent (27/43) of GATTEX-treated patients versus 30% (13/43) of placebo-treated patients were considered responders (p=0.002).
At Week 24, the mean reduction in weekly PN/I.V. volume was 4.4 Liters for GATTEX-treated patients (from pre-treatment baseline of 12.9 Liters) versus 2.3 Liters for placebo-treated patients (from pre-treatment baseline of 13.2 Liters/week) (p<0.001).
Twenty-one patients on GATTEX (54%) versus 9 on placebo (23%) achieved at least a one-day reduction in PN/I.V. support.
The mean changes from Baseline in PN/I.V. volume by visit are shown in Figure 2.
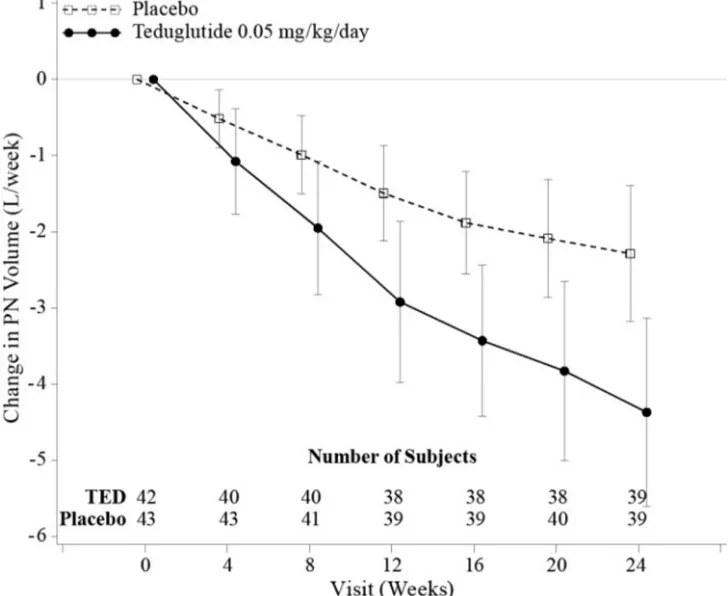
Figure 2: Change (±95% CI) in PN/I.V. volume (L/week)
Study 2 (CL0600-021, NCT00930644)
Study 2 was a 2-year open-label extension of Study 1 in which 88 patients received GATTEX 0.05 mg/kg/day. Ninety-seven percent (76/78) of patients who completed Study 1 elected to enroll in Study 2 (37 received GATTEX; 39 received Placebo). An additional 12 patients entered Study 2, who had been optimized and stabilized but not randomized in Study 1 because of closed enrollment.
30 months exposure
Thirty GATTEX patients completed a total duration of 30 months (Study 1 followed by Study 2 treatment). Of these, 28 patients (93%) achieved a 20% or greater reduction of parenteral support (PS). Of responders in Study 1 who had completed 2 additional years of continuous treatment with GATTEX, 96% (21/22) sustained their response to GATTEX. The mean reduction in PN/I.V. (n=30) was 7.55 L/week (a 66% reduction from baseline). Ten patients were weaned off their PN/I.V. support while on GATTEX treatment for 30 months. Patients were maintained on GATTEX even if no longer requiring PN/I.V. support. These 10 patients had required PN/I.V. support for 1.2 to 15.5 years, and prior to GATTEX had required between 3.5 L/week and 13.4 L/week of PN/I.V. support. At the end of study, 21 (70%), 18 (60%) and 18 (60%) of the 30 completers achieved a reduction of 1, 2, or 3 days per week in PN/I.V. support, respectively.
24 months exposure
Of the 39 placebo-treated patients from Study 1 entering Study 2, 29 completed 24 months of treatment with GATTEX. The mean reduction in PN/I.V. was 3.11 L/week (an additional 28.3% reduction) from the start of Study 2. Sixteen (55%) of the 29 completers achieved a 20% or greater reduction of PS. At the end of the study, 14 (48%), 7 (24%) and 5 (17%) achieved a reduction of 1, 2, or 3 days per week in PN/I.V. support, respectively. Two patients were weaned off their PN/I.V. support while on GATTEX. Of the 12 patients entering Study 2 directly, 6 completed 24 months of treatment with GATTEX. Similar effects were seen. One of the six patients was weaned off their PN/I.V. support while on GATTEX.
Study 3 (Placebo-controlled) and Study 4 (Blinded Uncontrolled Extension of Study 3)
Study 3 (CL0600-004, NCT00081458)
Study 3 was a randomized, double-blind, placebo-controlled, three parallel-group, multinational study in adults with SBS who were dependent on parenteral nutrition/intravenous (PN/I.V.) support for at least 12 months and required PN at least 3 times per week. After a period of optimization and stabilization similar to Study 1, patients were randomized to receive 24 weeks of one of the following treatment regimens: GATTEX 0.05 mg/kg/day (n=35), GATTEX 0.1 mg/kg/day (twice the recommended dose) (n=33), or placebo (n=16). GATTEX 0.1 mg/kg/day is not a recommended dosage [see Dosage and Administration (2.2)]. The treatment groups were compared using the intent-to-treat population of this study which was defined as all randomized patients who were administered at least one dose of study drug. This population contained one less patient in the 0.1 mg/kg/day dose group hence n=32 in this group for all analyses. The primary efficacy endpoint was a graded categorical score that did not achieve statistical significance for the high dose. Further evaluation of PN/I.V. volume reduction using the endpoint of response (defined as at least 20% reduction in PN/I.V. fluid from Baseline to Weeks 20 and 24) showed that 46% of patients on GATTEX 0.05 mg/kg/day responded versus 6% on placebo. Patients on GATTEX in both dose groups experienced a 2.5 L/week reduction in PS requirements versus 0.9 L/week for placebo at 24 weeks. Two patients in the GATTEX 0.05 mg/kg/day dose group were weaned off PS by Week 24.
Study 4 (CL0600-005, NCT00172185)
Study 4 was a blinded, uncontrolled extension of Study 3, in which 65 patients from Study 3 received GATTEX for up to an additional 28 weeks of treatment. Of responders in Study 3 who entered Study 4, 75% sustained response on GATTEX after one year of treatment. In the GATTEX 0.05 mg/kg/day dose group, a 20% or greater reduction of PS was achieved in 68% (17/25) of patients. The mean reduction of weekly PN/I.V. volume was 4.9 L/week (52% reduction from baseline) after one year of continuous GATTEX treatment. The patients who had been completely weaned off PN/I.V. support in Study 3 remained off PS through Study 4. During Study 4, an additional patient from Study 3 was weaned off PS.
14.2 Treatment of SBS in Pediatric Patients
Study 5 (TED-C14-006, NCT02682381)
Study 5 was a 24-week, multicenter study conducted in 59 pediatric patients aged 1 year through 17 years with SBS who were dependent on PS. Patients chose whether to receive GATTEX or standard of care (SOC). Patients who chose to receive GATTEX treatment were subsequently randomized in a double-blind manner to 0.025 mg/kg/day (n=24) or 0.05 mg/kg/day (n=26), while 9 patients enrolled in the SOC arm. Randomization to the GATTEX dose groups was stratified by age.
Patients treated with 0.05 mg/kg had a mean age of 6 years at baseline. The most common reasons for intestinal resection leading to SBS were gastroschisis (54%, 14/26), midgut volvulus (23%, 6/26), and necrotizing enterocolitis (12%, 3/26). Stoma was present in 19% (5/26) of patients, and the most common type was jejunostomy (80%, 4/5). The mean length of remaining small intestine was 47 (±28) cm (range: 9 to 120 cm). In the 25 patients who had remaining colon, the colon was in continuity in 22 patients. At baseline, the mean PS volume was 60 (±29) mL/kg/day (range: 24 to 133 mL/kg/day) [8 (±4) L/week (range: 3 to 19 L/week)] and mean PS infusion time was 7 (±1) days/week (range: 5 to 7 days/week) and 11 (±3) hours/day (range: 7 to 20 hours/day).
Results described in Table 3 correspond to the recommended GATTEX dosage of 0.05 mg/kg subcutaneously once daily.
Table 3: Summary of Efficacy Endpoints at Week 24* for Study 5 – Patients treated with GATTEX 0.05 mg/kg/day (N = 26)
| Efficacy Endpoints | Results |
|---|---|
| Reduction in PS volume of at least 20%, n/N (%) | 18/26 (69%) |
| Achieved enteral autonomy, n/N (%) | 3/26 (12%) |
| Reduction in PS infusion of ≥1 day/week, n/N (%) | 10/26 (38%) |
| Change in PS volume from baseline (mL/kg/day), mean (SD) and [mean% (SD)] | -23 (18) mL/kg/day [-42% (29%)] |
Results based on patient diary data, ITT population
Study 6 (SHP633-304, NCT02954458)
Study 6 was a prospective, open-label, long-term extension study of pediatric patients who completed Study 5. In the extension study, patients received additional treatment with GATTEX 0.05 mg/kg subcutaneously once daily if they deteriorated or stopped improving after discontinuation of prior GATTEX treatment. Of the 15 patients who initially responded in Study 5 and enrolled in Study 6, 13 patients (87%) required additional treatment with GATTEX. Efficacy results at the end of the first 24-week treatment period in Study 6 (total treatment for a mean of 40 weeks) were similar to those achieved at the end of 24 weeks treatment in Study 5. One additional patient treated with 0.05 mg/kg in Study 5 eventually achieved enteral autonomy during follow-up in Study 6.
GATTEX (teduglutide) for injection is supplied as 5 mg of teduglutide as a white, lyophilized powder for reconstitution in a sterile, single-dose glass vial with 0.5 mL Sterile Water for Injection in a single-dose prefilled syringe. The product to be dispensed is either a one-vial kit or a 30-vial kit.
One-vial kits are pre-assembled and ready to be used:
GATTEX 5 mg One-vial Kit (NDC 68875-0103-1):
Storage and Handling of One-Vial Kit
Prior to Dispensing:
After Dispensing by the Pharmacist:
GATTEX is also supplied in 30-vial cartons to be assembled by the dispensing pharmacist into a 30-vial kit by transferring the trays containing 30 vials from a Carton of Drug Vials into a Carton of Ancillary Supplies:
GATTEX 5 mg Carton of Drug Vials (NDC 68875-0101-2):
The final assembled 30-Vial Kit should contain the items:
GATTEX 5 mg Strength 30-Vial Kit (NDC 68875-0102-1):
Storage and Handling of 30-Vial Cartons and 30-Vial Kits
Prior to Dispensing:
After Dispensing by the Pharmacist:
Advise the patient to read the FDA-approved patient labeling (Medication Guide and Instructions for Use).
Acceleration of Neoplastic Growth
Advise adult patients and their caregivers that the patient will need to undergo clinical examinations, repeated colonoscopies and upper GI endoscopies (or alternate imaging) before and during treatment with GATTEX to monitor for the development of polyps and/or neoplasia of the GI tract. Advise pediatric patients and their caregivers that the patient will need to undergo clinical examinations, fecal occult blood testing before and during treatment and colonoscopies after 1 year of treatment, every 5 years thereafter while on continuous treatment with GATTEX or if they have new or unexplained gastrointestinal bleeding. Advise pediatric patients and their caregivers that an upper GI endoscopy will be performed if there is any new or unexplained blood in the stool [see Warnings and Precautions (5.1)].
Intestinal Obstruction
Advise patients and their caregivers to immediately contact their healthcare provider if they experience any symptoms suggestive of intestinal or stomal obstruction [see Warnings and Precautions (5.2)].
Biliary and Pancreatic Disease
Advise patients and their caregivers that laboratory assessments will be done periodically while on GATTEX to monitor for the onset or worsening of gallbladder, biliary and pancreatic disease, and to report immediately to their healthcare provider if they develop symptoms suggestive of cholecystitis, cholangitis, cholelithiasis or pancreatic disease [see Warnings and Precautions (5.3)].
Fluid Overload
Advise patients and their caregivers to immediately contact their healthcare provider if they develop fluid overload or symptoms of congestive heart failure while on GATTEX [see Warnings and Precautions (5.4)].
Fluid Imbalance
Advise patients and their caregivers of the risk of fluid and electrolyte imbalance with discontinuation of GATTEX, and to contact their healthcare provider if they develop symptoms suggestive of electrolyte imbalances [see Warnings and Precautions (5.4)].
Increased Absorption of Concomitant Oral Medication
Instruct patients and their caregivers to report to their healthcare provider any concomitant oral medications that they are taking in order to assess any potential for increased absorption during GATTEX treatment of those oral medications requiring titration or with a narrow therapeutic index [see Warnings and Precautions (5.5)].
Lactation
Advise women that breastfeeding is not recommended during treatment with GATTEX [see Use in Specific Populations (8.2)].
GATTEX® (Ga’-tex)
(teduglutide)
for injection, for subcutaneous use
5 mg per vial
This Instructions for Use contains information on how to inject GATTEX.
Read this Instructions for Use before you start using GATTEX and each time you get a refill. There may be new information. Your healthcare provider or nurse should show you how to prepare, measure your dose, and give your injection of GATTEX the right way.
If you cannot give yourself the injection:
Self-administration is not recommended in pediatric patients. In pediatric patients, GATTEX should be injected by:
Important information:
GATTEX kit
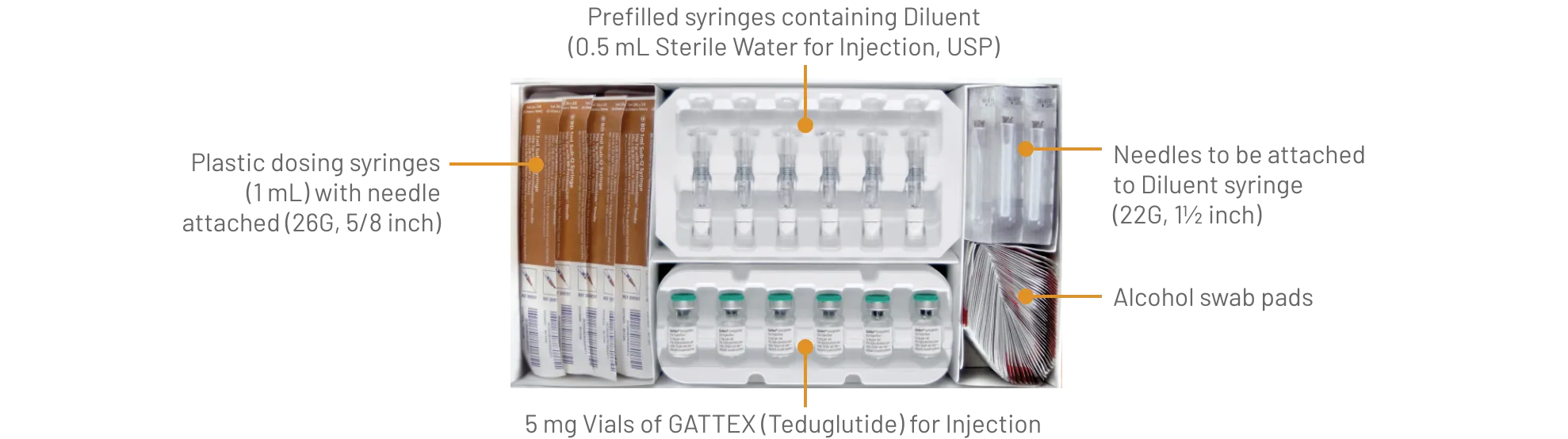
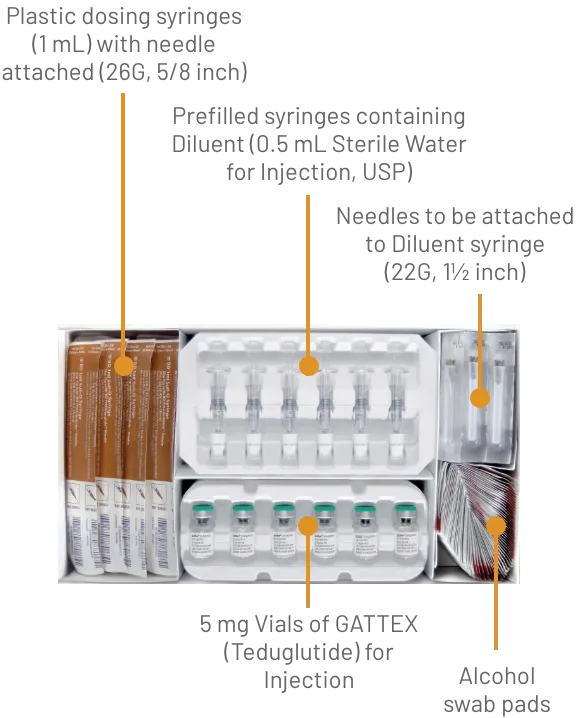
Gather the supplies you will need to prepare GATTEX and to give your injection (See Figure A).
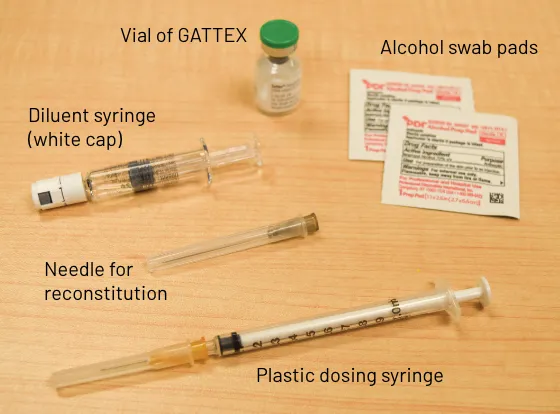
Figure A
From your GATTEX kit you will need:
You may also need an adhesive bandage (not included in your GATTEX kit).
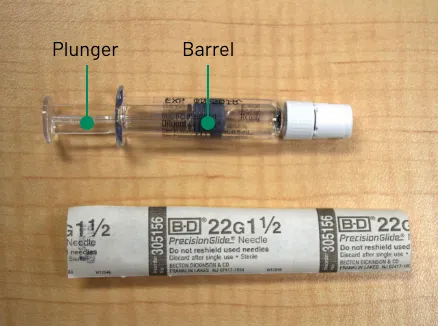
Figure B1
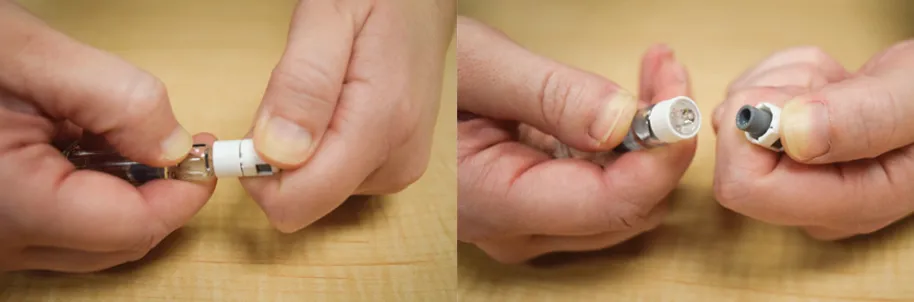
Figure B2

Figure C
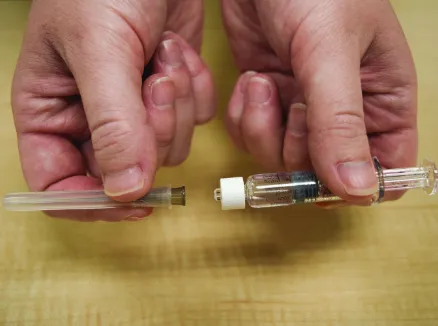
Figure D

Figure E

Figure F
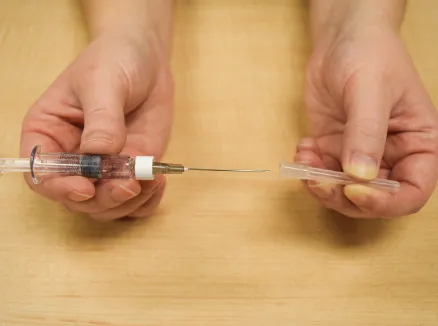
Figure G
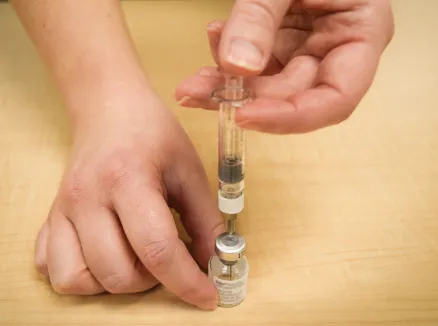
Figure H

Figure I

Figure J
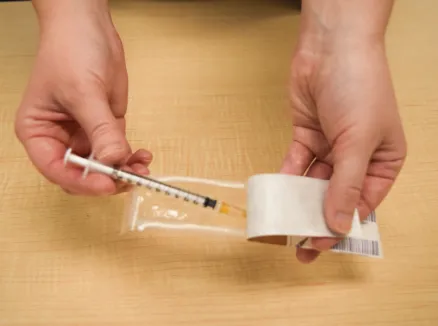
Figure K
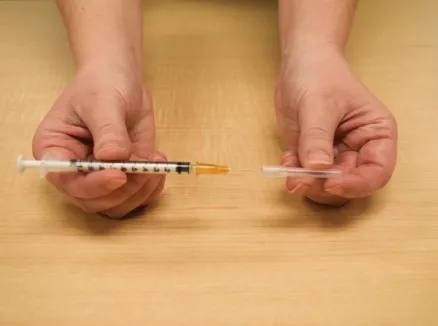
Figure L
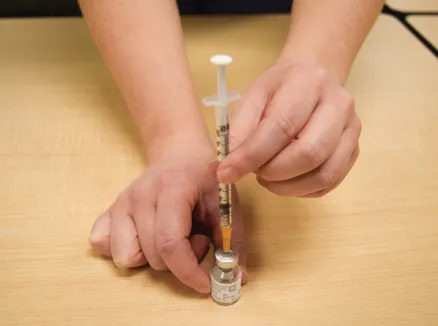
Figure M
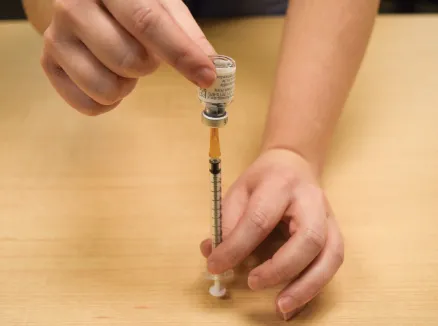
Figure N
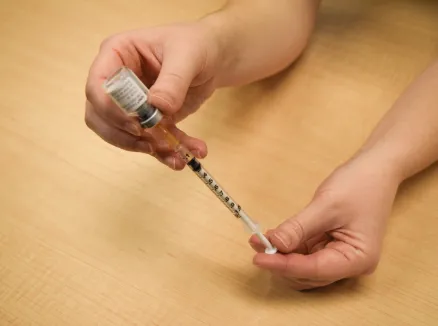
Figure O
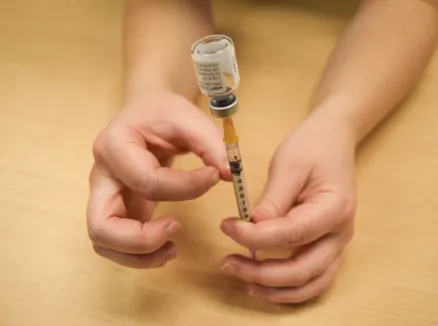
Figure P
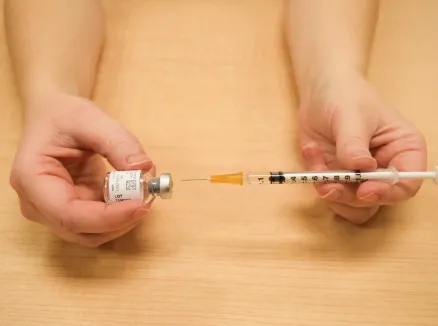
Figure Q
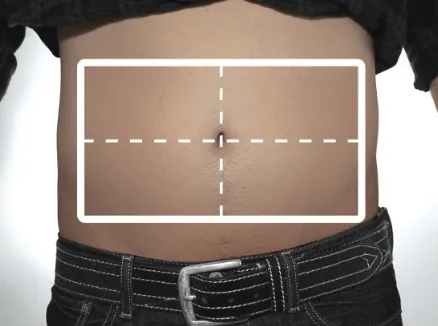
Figure R
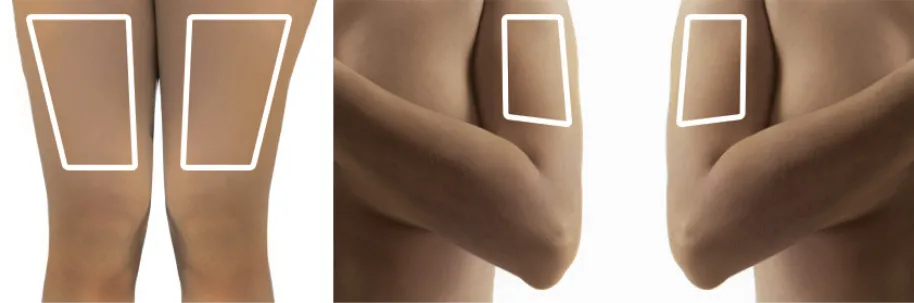
Figure S
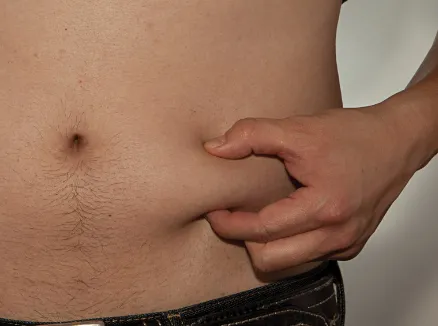
Figure T
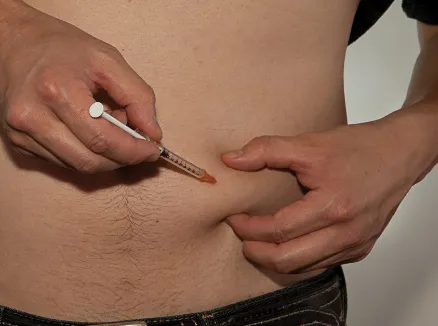
Figure U
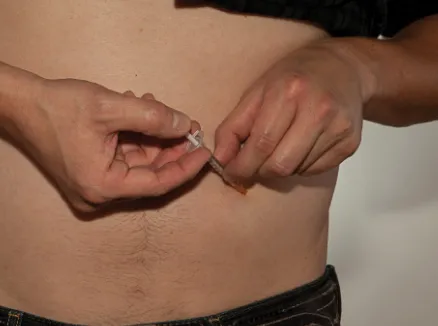
Figure V